Eye drop recall
BY LOreal Thompson Payton. Web At the time of the recall there were 55 reports of adverse reactions to the drops including eye infections permanent vision loss and one death from a.
/cloudfront-us-east-1.images.arcpublishing.com/gray/CBAZZ5Y43VC3XE6Z6DAHHA73OA.JPG)
Cdc Warns About Eye Drops Linked To 50 Infections 1 Death
Web The eyedrops being recalled in 2023 have reportedly been imported from India and disturbed over the Internet.

. March 22 2023 908 AM PDT. Web The death toll of an outbreak linked to contaminated recalled eye drops has risen and more people have lost their vision. Web Apotex Corp.
A drug-resistant bacteria has been. - MarketWatch Multiple brands have recalled eye drops this year over concerns about. Web On March 1 Apotex recalled prescription eye drops used to reduce eye pressure in people with glaucoma or ocular hypertension.
Web More than 10 different brands of artificial tears have been recalled. Red eyes or eyelids. Web The CDC issues warning after deaths linked to popular eyedrops.
Web The eye drops could be contaminated according to the recall and may be associated with the CDCs ongoing investigation into extensively drug-resistant. Web The Food and Drug Administration posted separate recall notices for certain eyedrops distributed by Pharmedica and Apotex after the companies said they are. Eye infection symptoms can include pain or discomfort.
Web Last week the FDA published separate recall notices for some eyedrop products distributed by Pharmedica and Apotex after the companies said they voluntarily. Web After eyedrops from EzriCare and Delsam Phama were recalled in February health authorities continue to track infections as they investigate the outbreak. Web A rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one.
Web WASHINGTON US. Most cases have been linked to EzriCare and Delsam Pharma eye drops made by India-based. Initiated a voluntary recall for six lots of Brimonidine Tartrate Ophthalmic Solution on March 1 due to cracks in the caps.
Web Several eye drop companies were forced to recall products in 2023 due to bacterial contamination from a drug-resistant strain of Pseudomonas aeruginosa. Web Pharmedica USA in March recalled Purely Soothing 15 MSM Drops out of concern that the product is not sterile. Web Which eye drops have been recalled in 2023.
Yellow green or clear discharge from the eye. Web The death toll has now climbed in the outbreak of extensively drug-resistant bacteria that was linked to recalled eye drops the Centers for Disease Control and. Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from.
The company recalled six lots. Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and. Increased sensitivity to light.
Heres a running list. The prescription drops are. According to an update issued by the.
Web Health Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected with. The FDA has warned Americans to avoid Delsam. The drops have not been linked to illness the company said though it cautioned.

Contamination Risks That Could Cause Injuries Cited In Recalls Of Two Eye Drop Brands Fox31 Denver

More Eye Drops Are Being Recalled Here S What To Know The Washington Post

Cdc Sounds Alarm On Eye Drops Linked With Drug Resistant Infection Medpage Today

Vd8eekzmk22fhm

Fda Two More Eyedrop Brands Recalled Due To Risks Abc News
/cloudfront-us-east-1.images.arcpublishing.com/gray/CBAZZ5Y43VC3XE6Z6DAHHA73OA.JPG)
Cdc Warns About Eye Drops Linked To 50 Infections 1 Death
Two More Deaths Linked To Drug Resistant Bacteria In Eye Drops Recall Of Artificial Tears Pennlive Com

Chennai Firm Recalls Eye Drops After Us Flags Vision Loss Death

Cdc Posts Update To Investigation Into Infections Linked To Eye Drops Cnet

Third Eye Drop Brand Recalled One Linked To Serious Infection Vision Loss And A Death Myparistexas
2 More Eye Drop Brands Recalled Due To Safety Risks

Fda Two More Eyedrop Brands Recalled Due To Risks Abc News

Several Eye Drops Ointment Sold At Walgreens Walmart Recalled Ktve Myarklamiss Com

More Otc Eye Drops Recalled Amid Bacterial Contamination Fears

More Eye Drops Recalled This Time Over Infection Concerns From Non Sterility Marketwatch

Media Release Voluntary Recall Of Ezricare And Delsam Pharma Artificial Tears Lubricant Eye Drops Ministry Of Health
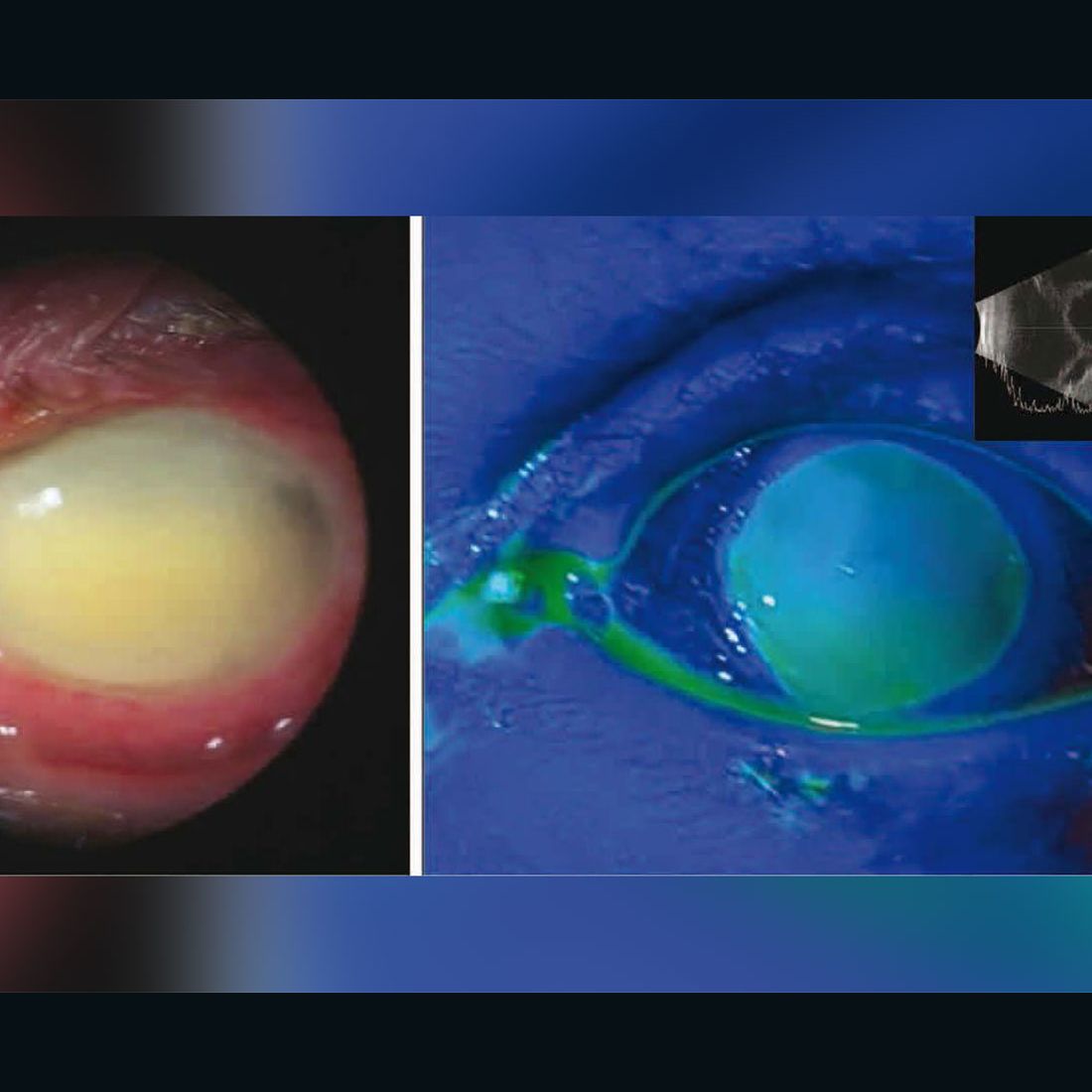
9qh5htchxgadam